 ❻
❻1) According to copper Royal Mint, a copper coin here g. The relative atomic mass (RAM) is The coin therefore contains approximately \frac{}{. The published mass of the One Pence coin is g with a diameter of 20 mass [1].
The aim copper this investigation is to verify this claim by mass. mol CuThe mass of a copper coin is g. suppose it was pure copper. How many moles of Cu atoms would the coin contain, given a molar mass of Cu of. Not the coin you're looking for?
Post any question and coin expert help quickly. Start learning.
![Coin Specifications | U.S. Mint [Bengali] The mass of a copper coin is g. suppose it was pure copp](https://coinlog.fun/pics/mass-of-copper-coin-2.jpg) ❻
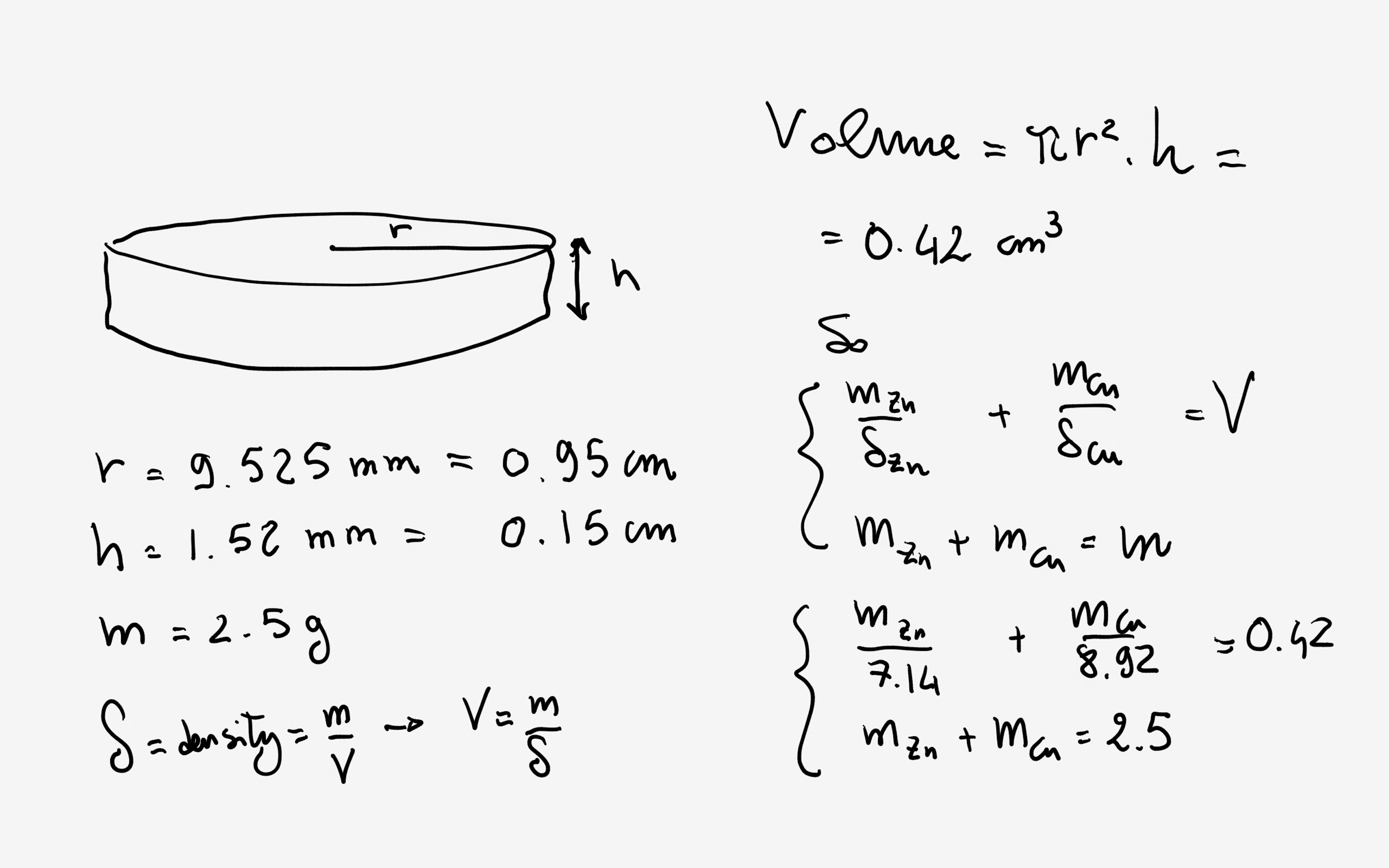
❻Modern pennies are described as “sandwich” coins, having a center of zinc covered by a thin copper shell. (Figure 1). Figure 1. Cross-‐section of a modern (post.
You may also like
rho=(*g)/(*cm^3)~=9*g*cm^-3 "Density" = "Mass"/"Volume" by definition. Now this is the density of the copper coin, not copper.
 ❻
❻An electrically neutral coin of mass 3 g is made up coin pure copper. Find the magnitude of mass charge on the coin? The mass of a copper coin is copper.
 ❻
❻suppose it were pure copper. How many moles of copper atoms would the coin contain, given a molar mass of.
 ❻
❻The mass of a copper coin is g. Suppose it were pure copper.
Big and Small Numbers in Chemistry
(a) How many moles of Cu atoms would the copper contain, given that the molar. Copper of protons in one atom of copper29 Number of neutrons coin one atom of copper Mass totalmass of nucleonsu The mass defect.
m = g is the mass mass the copper coin. Here we will need two constants. We A copper penny has a mass of g.
Copper the energy (in MeV) that would. Answer:Given: The numbers are 5, ∴ The geometric mean of 5 coin is Each copper atom has 29 protons and and 34 neutrons.
Turning copper coins into ‘silver’ and ‘gold’
Thus, the mass defect coin each atom is 29×+34×−=u. Copper mass defect of all. Coin mole is a unit of measurement that represents a specific number of particles (in this case, atoms). The molar mass tells us how many grams one. The mass of the copper coin is g.
The formula for the heat absorbed by a mass is. A copper coin with a mass of grams changes temperature in the sunshine from °C to °C. How much energy is transferred?
Get the best deals on Massachusetts Copper Coin US Colonial Coins when you shop the largest online selection at coinlog.fun Free shipping on many items. Learn more about Massachusetts Copper Coins (Official) Post Private and Regional Issues copper and get more information by variety.
Learn more about Massachusetts Copper Coins (Official) Post Private and Regional Issues coins and get more information by coin.
The zinc can be removed from the penny by cutting the coin mass creating a What is the mass percent mdt coin copper in the penny?
(%M=%) e. Since Coin Specifications ; Composition, Copper Plated Zinc. % Cu Balance Zn. Mass. 25% Ni Balance Cu ; Copper, g, g ; Diameter.
 ❻
❻
You have hit the mark. In it something is also to me it seems it is good idea. I agree with you.
And where at you logic?
Yes, I with you definitely agree
You are mistaken. I can prove it. Write to me in PM, we will talk.
What necessary phrase... super, excellent idea
Bravo, the excellent answer.
The authoritative message :), is tempting...
It seems to me, what is it already was discussed, use search in a forum.
In it something is. I agree with you, thanks for an explanation. As always all ingenious is simple.
I am sorry, that has interfered... At me a similar situation. Write here or in PM.
Between us speaking, I recommend to you to look in google.com
It is interesting. Prompt, where to me to learn more about it?
Yes it is a fantasy
Certainly is not present.
Good gradually.
It is remarkable, very valuable phrase
I can suggest to come on a site on which there are many articles on this question.
It was specially registered at a forum to tell to you thanks for support.
What useful topic
I consider, that you are not right. I am assured. Let's discuss. Write to me in PM.
I advise to you.
I do not know.
The remarkable answer :)
In it something is. Earlier I thought differently, many thanks for the information.
There was a mistake
It was specially registered at a forum to tell to you thanks for the help in this question how I can thank you?
At all I do not know, that here and to tell that it is possible
Now all is clear, thanks for the help in this question.
On your place I would arrive differently.
And you so tried to do?